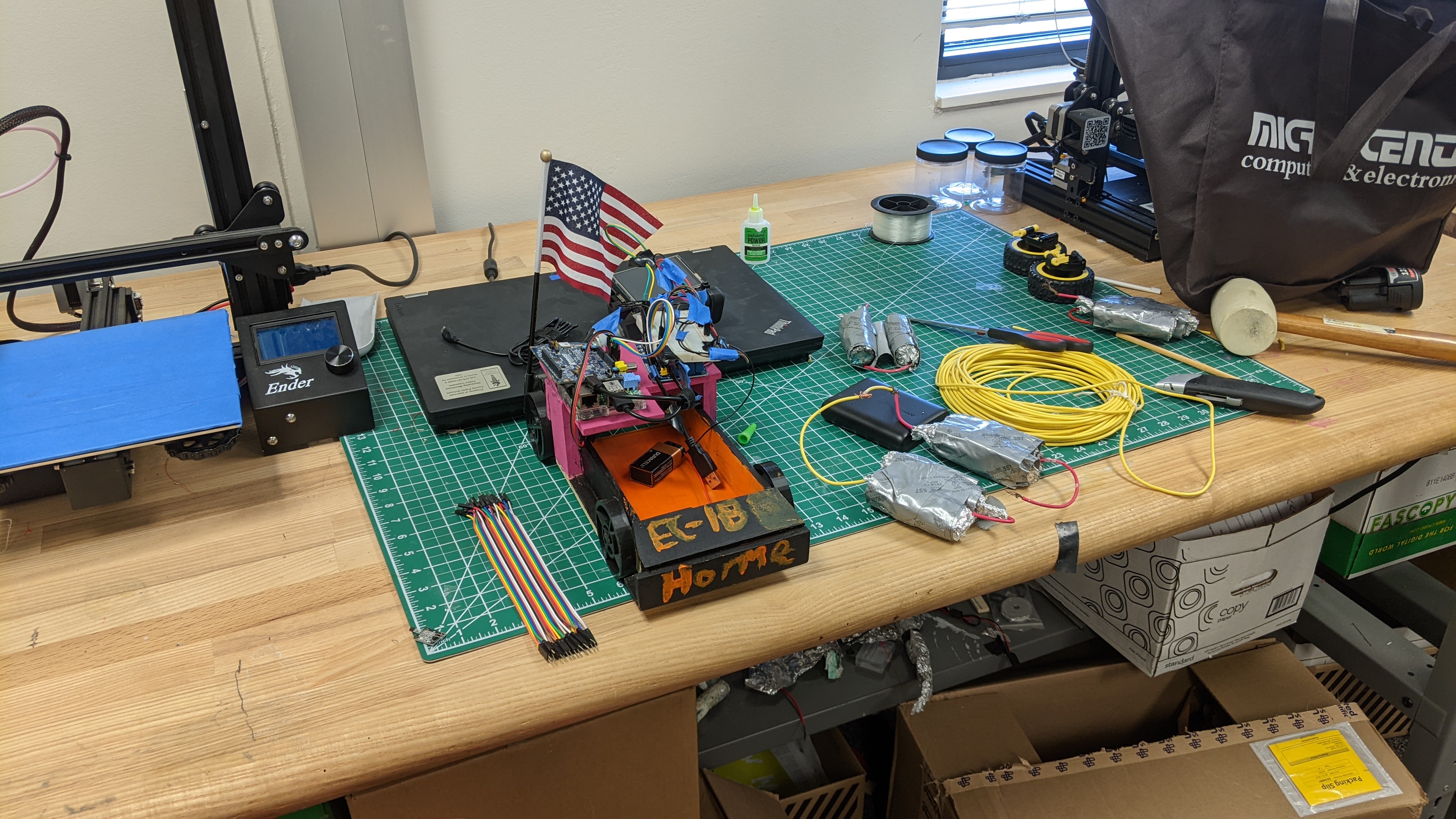
EC-1B Horme
This car project is basically just an upgraded version of the mouse trap car.

The Goal
The goal for this project was to construct a car powered by a chemical reaction that could stop at a given location.
Prior Research
Our group began by researching potential chemical reactions to use for our project. The objective was to use thermoelectric generators (TEGs) to power a motor, and we explored different components and reactions for the car. One member of the group focused on identifying an exothermic reaction to drive the TEGs, while I worked on researching the components to purchase, such as the motor, thermoelectric generators, heatsink, and so on. We used Discord to communicate our findings and ideas instantly. Initially, we thought of an acid-base neutralization reaction (HCl + NaOH), but after further research, we found that the reaction generated insufficient heat. We then considered other exothermic reactions, such as the combination of sulfuric acid and water. Eventually, we came across videos on YouTube about the decomposition of hydrogen peroxide, and after conducting further research with the group, we decided that it would be the best reaction to use. Additionally, we conducted research on where to purchase the necessary components.

Chemical Reactions
Catalyzed Decomposition of Hydrogen Peroxide:
2H₂O₂(aq) [MnO₂(s)] → 2H₂O(g) + O₂(g)
Combustion of Paraffin Wax:
C₃₁H₆₄(s) + 47O₂(g) → 32H₂O(g) + 31CO₂(g)
The natural decomposition of hydrogen peroxide into oxygen and water occurs due to its instability, however, a catalyst can accelerate this process. In the case of hydrogen peroxide, manganese dioxide serves as the catalyst for the decomposition reaction. This reaction generates heat along with the formation of oxygen and water as products. The produced oxygen is then utilized to enhance the combustion of paraffin wax, leading to the release of heat, water, and carbon dioxide.
The reason our group selected this particular chemical reaction is that we anticipate it will generate a greater amount of heat, which in turn can be used to power the thermoelectric generators positioned on the top of the reaction chamber. After watching several videos on constructing an oxygen generator, we observed how the additional oxygen impacted the combustion reaction. By increasing the amount of heat produced, we expect to generate more power through the thermoelectric generators, which will ultimately be transmitted to the motor responsible for turning the wheels.
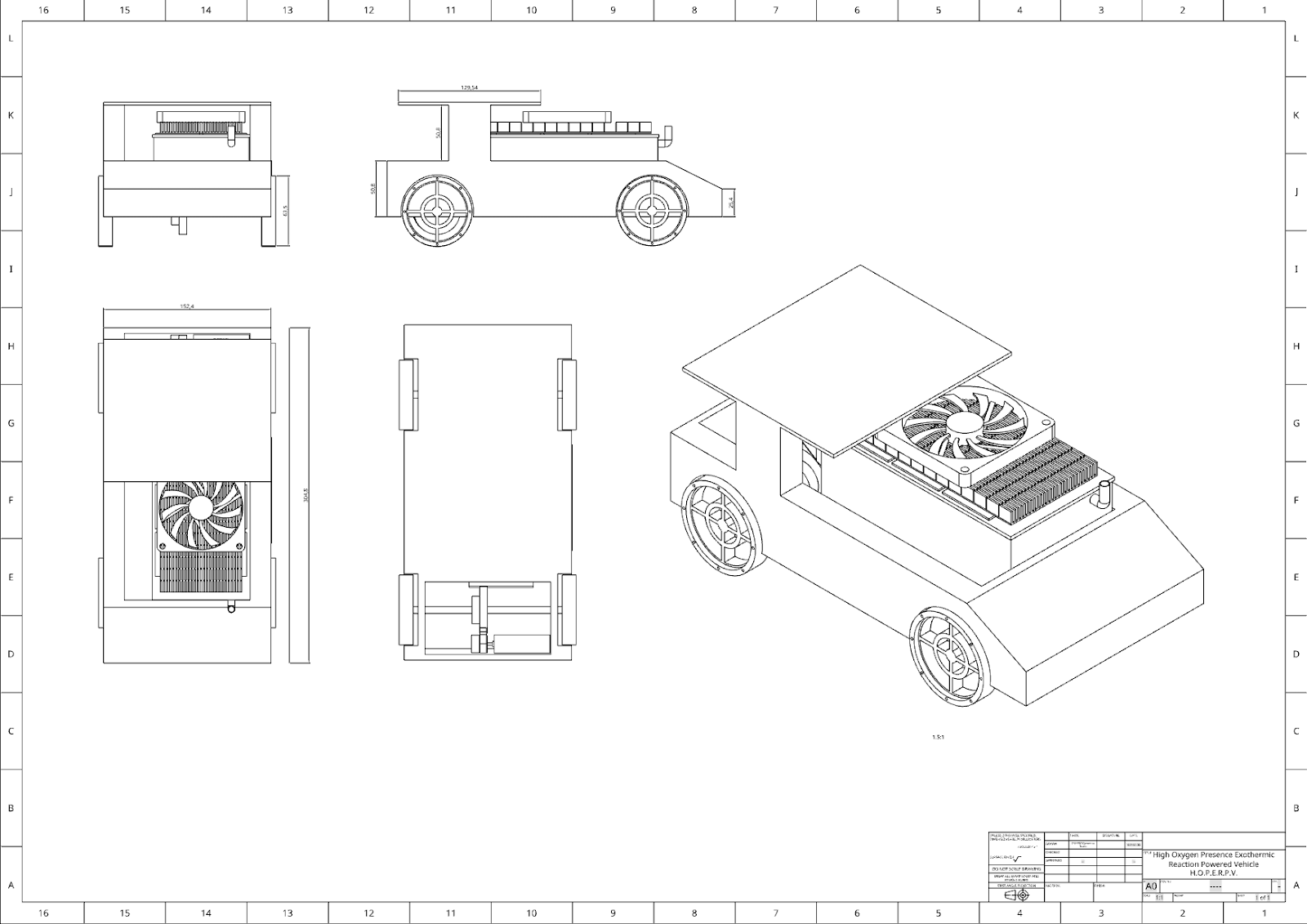
Planning
Following the completion of our research on the chemicals and components to be used for the car, our group proceeded to the planning phase. We began by creating a preliminary model of the car using Onshape software, which we shared with the group for feedback. Ryan was responsible for developing the design and dimensions of the reaction chamber, and Toan modeled it in Onshape. Within two days of being assigned the project, we had a clear concept of what we wanted to construct. Toan then created an updated version of the car model with accurate dimensions and wheels.
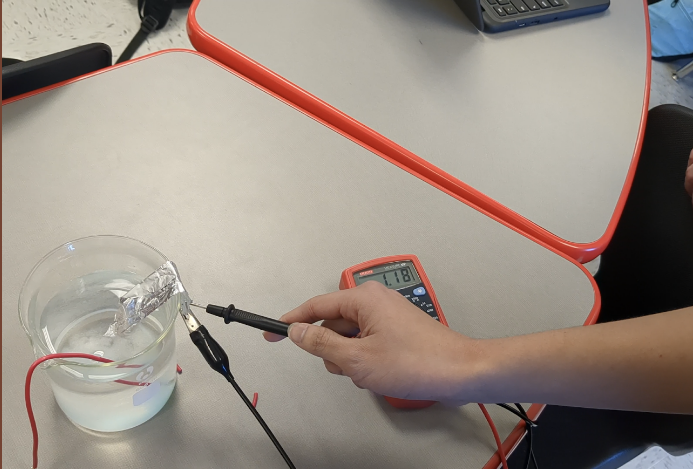
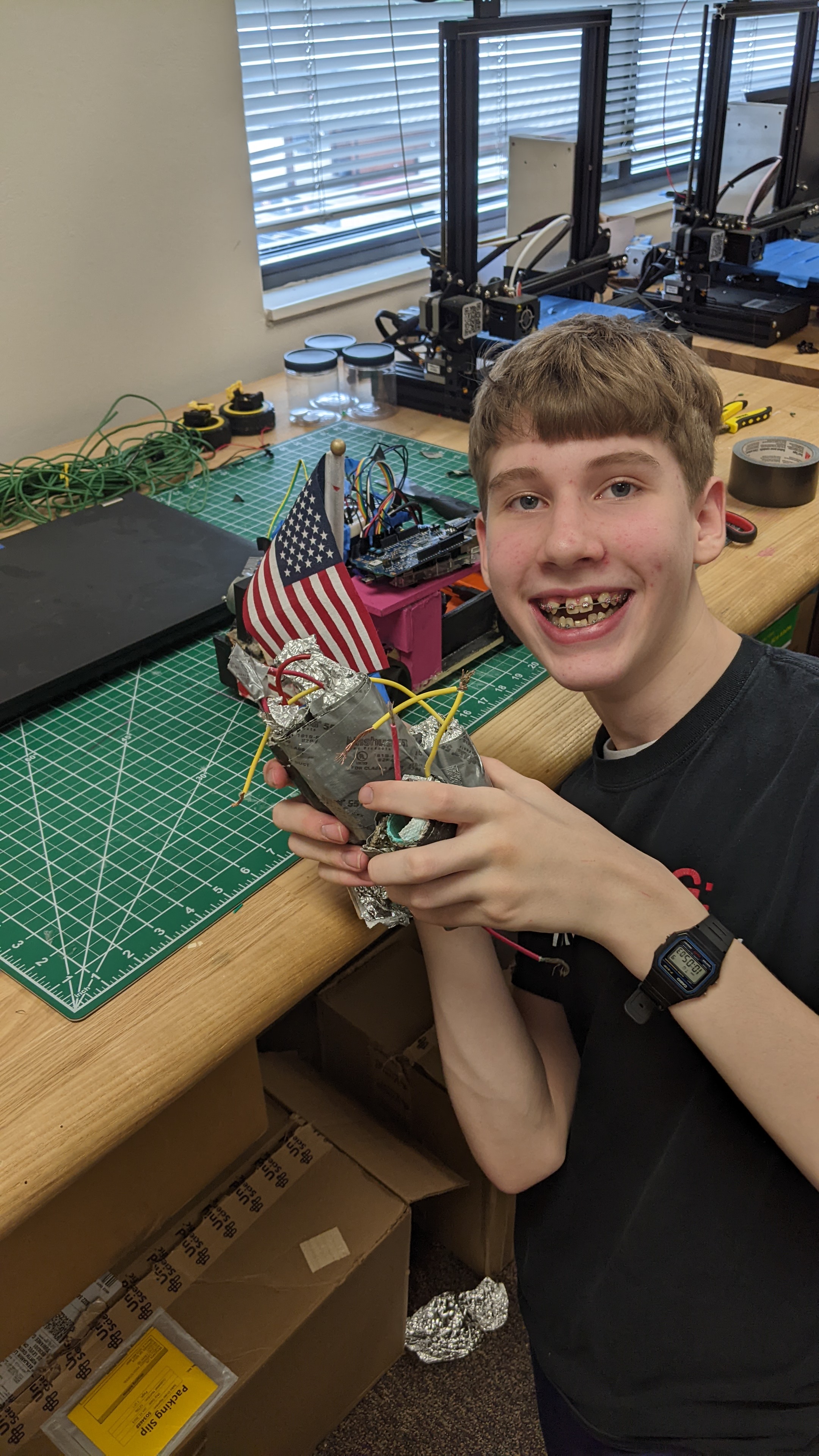
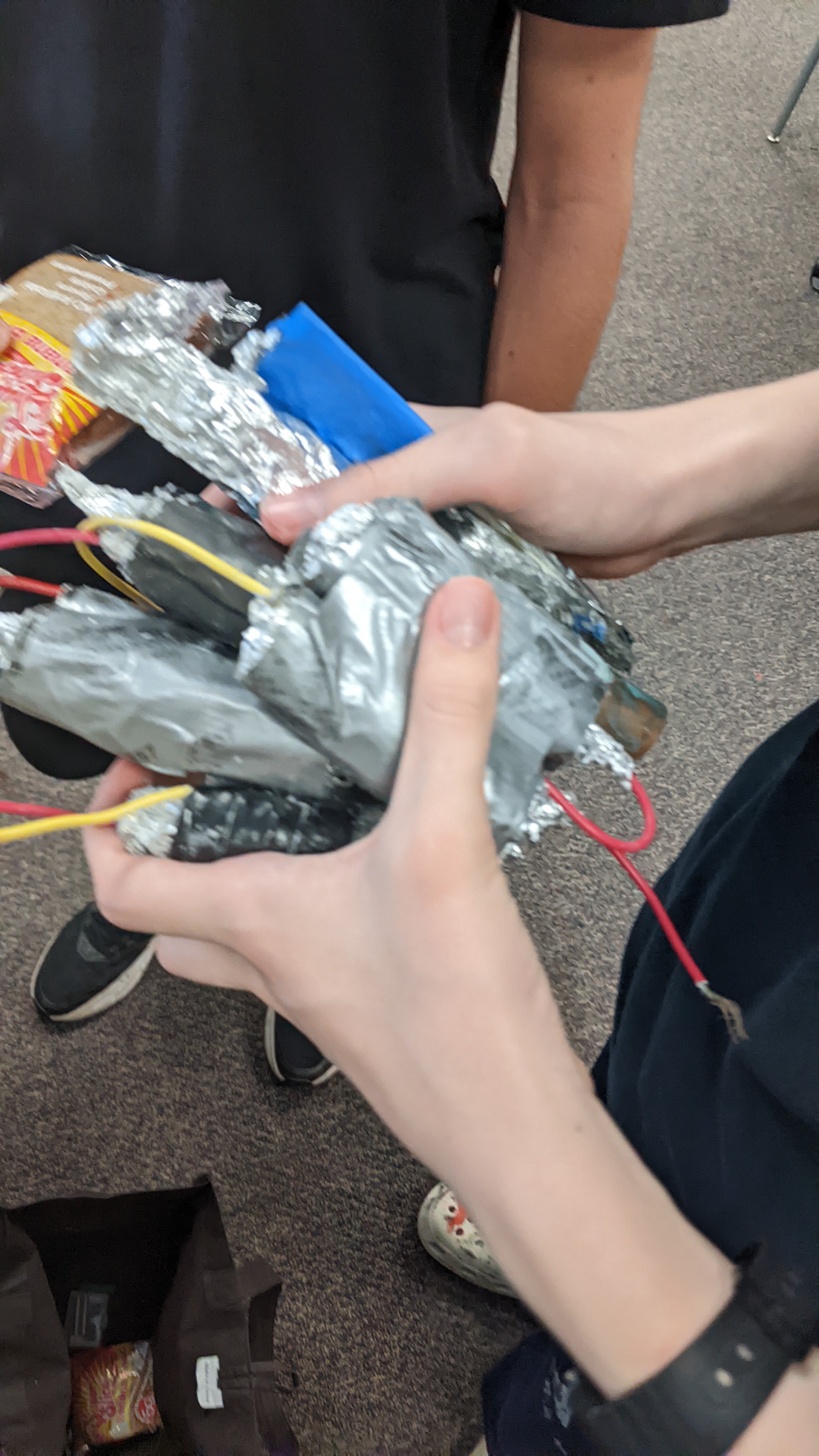
Batteries
One of our main ideas was to store power in homemade batteries. The chemical battery works through electrolysis between a sodium chloride water solution and a copper pipe. Above is an example of how it would work just with a beaker, producing at least a volt of output per battery (which weren't very concentrated). This would provide enough power to use the motor we had chosen.

TEGs
Our second idea was to provide additional power using Thermo Electric Generators (TEGs). These generate electricity from a difference in heat on one side compared to the other. To heat the TEGs we used candles boosted with oxygen from a hydrogen dioxide and manganese dioxide reaction.

Stopping Mechanism
A critical feature of the car will be its capability to come to a stop on its own. This will be accomplished through the implementation of a braking system controlled by a controller and a Raspberry Pi 1B, both of which will be developed by our highly skilled coders here at CHARGE Dynamics. By programming a particular distance into the system, the motor will discontinue operation once that distance has been achieved, effectively bringing the car to a stop.
Construction

Body - Angle 1

Body - Angle 2

Wheels

Minor Issue
After a small chemical outburst in the lab, Manganese Dioxide, the main chemical for our reaction, was banned, meaning that we would need to use another way to power the car. We decided to use chemical batteries. There was just one problem. With the chemicals allowed, we would need at least 8 batteries to power the car. (Picture shows the banned chemical list.)
Building the batteries:

Painting the Car
The painting of the car was quite random. We just used the paints that were in front of us at the time. Even so, I think the car turned out fairly well.

Final Design
The last change we made to this project was adding a slice of banana bread to the front for 'armor' and good luck.
Electronics

The vehicle utilizes a motor to drive the wheels using electricity as its power output. However, if the objective was solely to move through a chemical reaction, a thermoelectric generator could be connected to a motor. To stop the car, the motor is simply stopped once the desired distance is reached. The Raspberry Pi 1B onboard the car includes a magnet sensor that detects a magnet placed on the wheel, allowing the Pi to determine the distance traveled. A transistor connected to one of the GPIO pins on the Pi 1B toggles the power output of the thermoelectric generator to control the motor. To wirelessly interact with the Pi 1B and determine the distance needed to be traveled, a Raspberry Pi 3B with a screen is contained within an enclosure that utilizes a R41Z Bluetooth-LE evaluation kit.
GitHub links for all the code:
R41Z Bluetooth-LE server
Bluetooth to serial client bridge